Click on images
to enlarge


Photographer: B.R. Maslin

Phyllodes broader than normal. Photographer: B.R. Maslin

Photographer: B.R. Maslin

Photographer: J. Maslin
, LSWE 7026, FM_sml.jpg)
Seed from one herbarium voucher. Scale in mm. Photographer: F. McCallum
Botanical name
Acacia adsurgens Maiden & Blakely, J. Roy. Soc. W. Australia, 13: 28, pl. 20, figs 6-10 (1928)
Aboriginal name
Pilarri (Kurrama)
Description
Spreading shrubs mostly 1-4 m tall, young plants often bushy and rounded but become openly branched, sometimes spindly and with an untidy aspect with age, dividing at ground level into a number of slender main, ascending to erect stems (maximum basal stem diameter is 10-15 cm). Bark grey to reddish brown, mostly smooth but may be rough and somewhat fibrous at base of main stems on oldest plants. Branchlets glabrous, apically resinous (but not sticky), light brown or reddish brown. New shoots green, resinous (but not sticky). Phyllodes linear, mostly 11-18 (-20) cm long, occasional phyllodes on some specimens only 8 cm, normally (1.5-) 2-4 mm wide but occasionally 5-7 mm, ascending to erect or wide-spreading, coriaceous, straight or shallowly incurved, resinous (at least when young) but not sticky, glabrous, greyish green, sub-glaucous or dull green (usually pale yellowish green or milky green when dry); parallel longitudinal nerves numerous, fine and close together, the central nerve slightly more evident than the rest. Inflorescences simple; peduncles 5-15 mm long (normally about the same length as the spikes), glabrous, resinous but not sticky; spikes shortly cylindrical, 10-20 mm long when dry, pale yellow to light golden, flowers close together producing a dense spike. Flowers 5-merous; calyx exceeding ½ length of petals, shortly dissected into broadly triangular lobes. Pods quite prolific, linear, flat to ±terete, scarcely constricted between seeds, 4-12 cm long, 2-3 mm wide, firmly chartaceous to thinly crustaceous, straight or shallowly curved, sweetly perfumed, light brown or greyish brown and slightly mealy white. Seeds longitudinal in the pods, narrowly obloid to obloid-ellipsoid, 3.5-4.5 mm long, about 2 mm wide, seeds remain dangling from pod for a short time after dehiscence, moderately shiny, very dark brown to blackish, dull yellow at centre around the small areole; aril rather large, convoluted and bright yellow.
Characteristic features
Spreading, multi-stemmed, glabrous shrubs. Phyllodes linear, long and narrow (mostly 11-18 cm x 2-4 mm wide), usually pale yellowish green or milky green when dry, with numerous, very fine, parallel, longitudinal nerves, the central nerve slightly more pronounced than the others. Spikes relatively short (10-20 mm long), pale yellow, densely flowered; peduncles often about as long as the spikes. Calyx exceeding ½ length of petals, shortly dissected. Pods linear, long and narrow (4-12 cm x 2-3 mm), thin-textured. Seeds with a yellow, convoluted aril.
Distribution and ecology
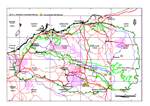
Widespread but not especially abundant in northern arid regions (between 18 degrees S and 26 degrees S) where it extends from the Pilbara region eastwards through Western Australia, Northern Territory and far northeast South Australia to central Queensland. Acacia adsurgens has a scattered but reasonably widespread distribution in the Pilbara where it extends from near Wittenoom to east of Nullagine and Newman. It grows with spinifex on flats and rocky hillsides, often along watercourses. Acacia adsurgens regenerates from seed following fire or other disturbance and can form rather dense, localized thickets, especially where water is not limiting. Over its entire geographic range A. adsurgens typically grows in deep, well-drained, infertile, acidic, red sand and sandy loam on sandplains, interdunal plains and sandy rises; it may also occur in skeletal soil in rocky habitats such as gravelly scree slopes. It is normally found in open communities with a spinifex-dominated ground cover. The above information is taken from Thomson and Hall (1989) and Doran and Turnbull (1997) who provide additional habitat information.
Flowering and fruiting period
Flowering has been recorded from April to September with the main flush occurring from May to July. Acacia adsurgens appears to have quite a long flowering period with spikes generally not produced in great profusion; often inflorescence buds, spikes at anthesis and young pods are present on plants at the same time. Pods with mature seeds have been collected from September to November. According to Latz (1999) seed production depends upon rainfall (presumably its incidence and intensity). The seeds are produced in moderate quantities (heavy seed crops are produced in good seasons); typically each spike produces many pods.
Variation
Relatively invariate within the Pilbara although the occasional specimen may have phyllodes to about 5-7 mm wide.
Affinities
Most closely related to A. tenuissima , with which it sometimes grows but which is most readily distinguished by having slender, terete phyllodes. Superficially A. adsurgens may be confused with the long, linear phyllode form of A. sibirica which is distinguished by generally shorter phyllodes (mostly 40-90 mm long) that are uniformly finely nerved (the central nerve is not more pronounced as occurs on A. adsurgens), less densely flowered spikes (with the flowers smaller and the calyx shorter relative to the corolla) and broader pods, also the spikes of A. sibirica are commonly about twice as long as the peduncles whereas in A. adsurgens the peduncles often about as long as the spikes. Acacia adsurgens may possibly hybridize with A. rhodophloia in the central Hamersley Range area (see A.? adsurgens x rhodophloia). Also related to an entity of uncertain taxonomic status that occurs east of Newman (see Acacia sp. Jimblebar (S. van Leeuwen 1342).
Notes
Acacia adsurgens appears to be a moderately fast-growing but relatively short-lived species (plants have been reported to reach 1 m tall and attain flowering 22 months after fire in Central Australia, and then become senescent at about 8 years, P. Latz pers. comm., cited in Thomson and Hall 1989), with a moderate coppicing ability.
The wood is dense (1030 kg/m3) with a dark brown heartwood and a narrow band of pale sapwood and is suitable for firewood and has excellent characteristics for conversion into charcoal (although its small size would limit its use for this purpose, see Thomson and Hall 1989).
It is reported to be palatable to cattle (Chippendale 1958) but does not appear to be grazed to any extent in its native habitat. Although the phyllodes may contain low levels of cyanogenic glycoside the species is unlikely to be dangerous to stock (Maslin et al. 1987).
This species has some potential for planting as low shelter belts and sand stabilization.
The seeds of A. adsurgens have been used traditionally as a food source by aborigines (O'Connell et al. 1983, Latz 1999), who roasted and ground the seeds to a paste prior to consumption. The Warlpiri people of Central Australia have used the foliage of this species for medicinal purposes: the phyllodes are boiled and used as a wash for general complaints, or used to smoke babies as a treatment for diarrhea (Latz 1999). A source of edible grubs (Bilu) for the Kurrama people of the central Pilbara (Lola Young 2007).
Further information on the utilisation and silviculture of this species is given in Thomson and Hall (1989) and Doran and Turnbull (1997); Latz (1995 and 1999) provides details of Aboriginal usage.
The seeds with their brightly coloured arils dangling from pods for a short time following dehiscence is presumably a bird-attractant.
Conservation status
Not considered rare or endangered.
Origin of name
The botanical name is derived from the Latin adsurgens (ascending or rising up), presumably in reference to the erect orientation of the phyllodes on the specimens that the species authors (Maiden and Blakey 1928) used to prepare the original description of the species. However, as can be seen from the above description, the phyllodes are commonly wide-spreading.
References
Chippendale, G.M. (1958). Notes on the vegetation of a desert area in central Australia. Transactions of the Royal Society of South Australia 81: 31-42.
Doran, J.C. and Turnbull, J.W. (1997). Australian trees and shrubs: species for land rehabilitation and farm planting in the tropics. ACIAR Monograph No. 24. pp. 384. (Australian Centre for International Agricultural Research: Canberra.)
Latz, P.K. (1995). Bushfires and Bushtucker: Aboriginal plant use central Australia. pp. 400. (IAD Press: Alice Springs.)
Latz, P.K. (1999). Pocket Bushtucker: a field guide to the plants of Central Australia and their traditional uses. pp. 215. (IAD Press: Alice Springs.)
Maiden, J.H. and Blakely, W.F. (1928). Descriptions of fifteen new Acacias and notes on several other species. Journal and Proceedings of the Royal Society of New South Wales 60: 171-196.
Maslin, B.R., Conn, E.E. and Dunn, J.E. (1987). Cyanogenic Australian species of Acacia: a preliminary account of their toxicity potential. pp. 107-111. In: J.W. Turnbull (ed.) Australian Acacias in developing countries. Proceedings of an international workshop held at the Forestry Training Centre, Gympie, Australia, 4-7 August 1986. ACIAR Proceedings No. 16. pp. 196. (Australian Centre for International Agricultural Research: Canberra.)
O'Connell, J.F., Latz, P.K. and Barnett, P. (1983). Traditional and modern plant use among the Alyawara of central Australia. Economic Botany 37(1): 80-109.
Thomson, L.A.J. and Hall, N. (1989). Acacia adsurgens. Australian Acacias, Leaflet No. 22 (CSIRO Division of Forests and Forest Products: Canberra.)
Young, L. (2007). Lola Young: Medicine Woman and Teacher. Complied by Anna Vitenbergs. pp. 160. (Fremantle Arts Centre Press: Fremantle.)